Journal Description
Clinics and Practice
Clinics and Practice
is an international, scientific, peer-reviewed, open access journal on clinical medicine, published bimonthly online by MDPI (from Volume 11, Issue 1 - 2021).
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, Embase, and other databases.
- Journal Rank: CiteScore - Q2 (General Medicine)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 26.4 days after submission; acceptance to publication is undertaken in 3.8 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Impact Factor:
2.3 (2022);
5-Year Impact Factor:
2.0 (2022)
Latest Articles
Clinical Approach to Patients with COVID-19 and Unrecognized Obstructive Sleep Apnea
Clin. Pract. 2024, 14(2), 629-641; https://doi.org/10.3390/clinpract14020050 - 18 Apr 2024
Abstract
►
Show Figures
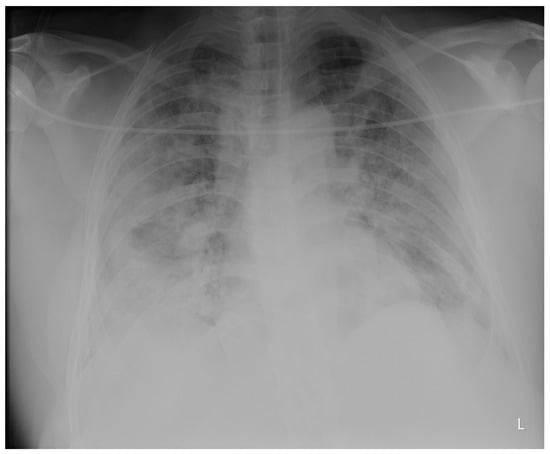
Purpose: We conducted a retrospective case series of seven male COVID-19 patients with respiratory failure and suspected OSA based on clinical features to evaluate the effects of undiagnosed obstructive sleep apnea (OSA) on COVID-19 outcomes and the response to a continuous positive airway
[...] Read more.
Purpose: We conducted a retrospective case series of seven male COVID-19 patients with respiratory failure and suspected OSA based on clinical features to evaluate the effects of undiagnosed obstructive sleep apnea (OSA) on COVID-19 outcomes and the response to a continuous positive airway pressure (CPAP) treatment. Cardiorespiratory polygraphy (CRP) and a continuous positive airway pressure treatment were used for diagnosis and management. They confirmed severe obstructive sleep apnea in all patients (apnea/hypopnea index > 30) and improved overnight oxygenation and symptoms at the 1-month follow-up. Conclusions: Undiagnosed obstructive sleep apnea may negatively impact COVID-19 outcomes by exacerbating respiratory failure. Recognition and treatment with continuous positive airway pressure can optimize the management of such patients.
Full article
Open AccessCase Report
Successful Interventional Endovascular Management of Ruptured Penetrating Aortic Ulcer with Associated Enormous Right Pleural False Aneurysm
by
Andrei Emanuel Grigorescu, Andrei Anghel and Horea Feier
Clin. Pract. 2024, 14(2), 619-628; https://doi.org/10.3390/clinpract14020049 - 17 Apr 2024
Abstract
►▼
Show Figures
Penetrating aortic injuries represent critical medical emergencies that necessitate immediate intervention to prevent life-threatening consequences. When accompanied by the presence of an enormous right pleural false aneurysm, the clinical scenario becomes exceptionally rare and complex. This case report details the successful management of
[...] Read more.
Penetrating aortic injuries represent critical medical emergencies that necessitate immediate intervention to prevent life-threatening consequences. When accompanied by the presence of an enormous right pleural false aneurysm, the clinical scenario becomes exceptionally rare and complex. This case report details the successful management of a patient who presented with a penetrating aortic ulcer and an extensive false aneurysm within the right pleura, employing an interdisciplinary approach involving cardiac surgeons, cardiologists, interventional cardiologists, and radiologists. The pivotal intervention involved the deployment of a covered and bare stent graft into the descending thoracic aorta to seal the aortic rupture. The patient’s clinical condition stabilized postoperatively, with no signs of recurrent hemorrhage. This case underscores the importance of rapid diagnosis, timely intervention, and the collaborative efforts of a specialized medical team in successfully managing such complex vascular injuries. Early recognition and referral to specialized centers are essential for improving patient outcomes in cases of penetrating aortic injuries with associated giant pseudoaneurysms.
Full article
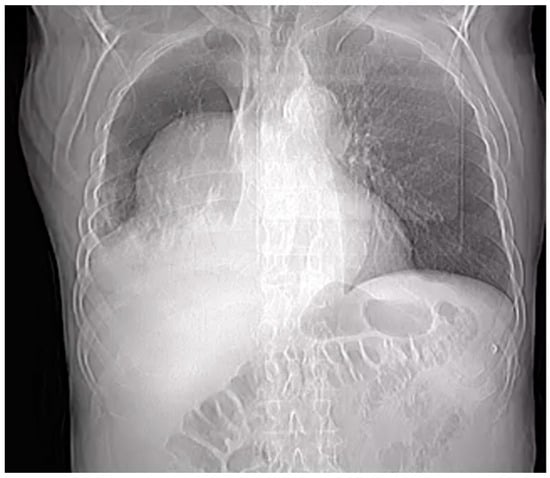
Figure 1
Open AccessOpinion
“Every Cloud Has a Silver Lining”: How Three Rare Diseases Defend Themselves from COVID-19 and What We Have Learnt from It
by
Martina Cacciapuoti, Ilaria Caputo, Lucia Federica Stefanelli, Paul A. Davis, Federico Nalesso and Lorenzo A. Calò
Clin. Pract. 2024, 14(2), 614-618; https://doi.org/10.3390/clinpract14020048 - 08 Apr 2024
Abstract
►▼
Show Figures
The process of SARS-CoV-2 infection, responsible for the COVID-19 pandemic, is carried out through different steps, with the interaction between ACE2 and Spike protein (S) being crucial. Besides of that, the acidic environment of endosomes seems to play a relevant role in the
[...] Read more.
The process of SARS-CoV-2 infection, responsible for the COVID-19 pandemic, is carried out through different steps, with the interaction between ACE2 and Spike protein (S) being crucial. Besides of that, the acidic environment of endosomes seems to play a relevant role in the virus uptake into cells and its intracellular replication. Patients affected by two rare genetic tubulopathies, Gitelman’s and Bartter’s Syndromes, and a rare genetic metabolic disease, Fabry Disease, have shown intrinsic protection from SARS-CoV-2 infection and COVID-19 on account of specific intrinsic features that interfere with the virus uptake into cells and its intracellular replication, which will be reported and discussed in this paper, providing interesting insights for present and future research.
Full article
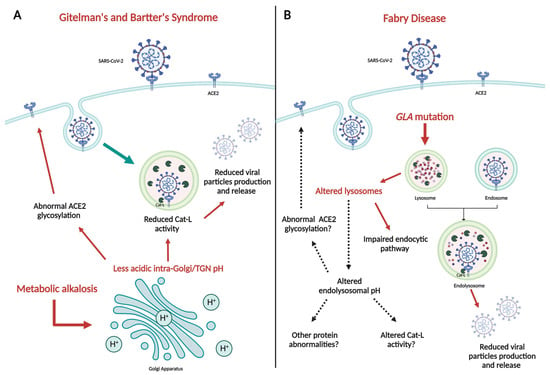
Figure 1
Open AccessArticle
Do MRI Results Represent Functional Outcomes Following Arthroscopic Repair of an Isolated Meniscus Tear in Young Patients?—A Prospective Comparative Cohort Study
by
Viktorija Brogaitė Martinkėnienė, Donatas Austys, Andrius Šaikus, Andrius Brazaitis, Giedrius Bernotavičius, Aleksas Makulavičius, Tomas Sveikata and Gilvydas Verkauskas
Clin. Pract. 2024, 14(2), 602-613; https://doi.org/10.3390/clinpract14020047 - 01 Apr 2024
Abstract
►▼
Show Figures
Background: The use of postoperative MRI to assess the healing status of repaired menisci is a long-standing issue. This study evaluates and compares functional and MRI outcomes following an arthroscopic meniscus repair procedure with the aim of postoperative MRI diagnostic accuracy clarification in
[...] Read more.
Background: The use of postoperative MRI to assess the healing status of repaired menisci is a long-standing issue. This study evaluates and compares functional and MRI outcomes following an arthroscopic meniscus repair procedure with the aim of postoperative MRI diagnostic accuracy clarification in young patients. Methods: A total of 35 patients under 18 years old who underwent isolated meniscus repair were included. The Pedi-IKDC score, Lysholm score, and Tegner activity index (TAS) were compared between the groups formed according to the Stroller and Crues three-grade classification of postoperative MRI-based evaluations. Grade 3 MRI views were classified as unhealed, grade 2 as partially healed, and grade 1 as fully healed within the repaired meniscus, whereas grade 3 cases were considered unsuccessful due to MRI evaluation. Results: MRI assessment revealed 4 cases of grade 1 (11.4%), 14 cases of grade 2 (40.8%), and 17 cases of grade 3 (48.0%) lesions. Pedi-IKDC and TAS scores were significantly higher among MRI grade 2 patients than among MRI grade 3 patients (p < 0.05). Weak negative correlations between MRI grades and all functional scales were found (p < 0.05). ROC analysis showed that Pedi-IKDC and TAS scores could correctly classify 77% and 71% of MRI grade 3 patients, respectively. The optimal cut-off values to detect grade 3 patients were 88.74 for the Pedi-IKDC score and 4.5 for the TAS score. Conclusions: To conclude, established functional score cut-off values may help identify unhealed meniscus repair patients.
Full article
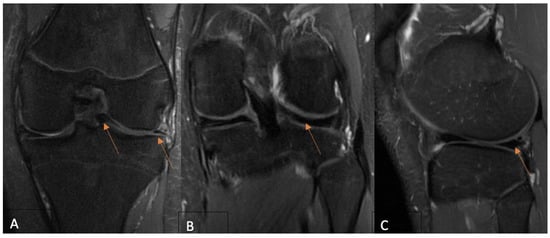
Figure 1
Open AccessArticle
Evaluating Global and Temporal Trends in Pancreas and Islet Cell Transplantation: Public Awareness and Engagement
by
Oscar A. Garcia Valencia, Charat Thongprayoon, Caroline C. Jadlowiec, Shennen A. Mao, Napat Leeaphorn, Pooja Budhiraja, Nadeen Khoury, Pradeep Vaitla, Supawadee Suppadungsuk and Wisit Cheungpasitporn
Clin. Pract. 2024, 14(2), 590-601; https://doi.org/10.3390/clinpract14020046 - 29 Mar 2024
Abstract
►▼
Show Figures
Background: Pancreas transplantation is a crucial surgical intervention for managing diabetes, but it faces challenges such as its invasive nature, stringent patient selection criteria, organ scarcity, and centralized expertise. Despite the steadily increasing number of pancreas transplants in the United States, there is
[...] Read more.
Background: Pancreas transplantation is a crucial surgical intervention for managing diabetes, but it faces challenges such as its invasive nature, stringent patient selection criteria, organ scarcity, and centralized expertise. Despite the steadily increasing number of pancreas transplants in the United States, there is a need to understand global trends in interest to increase awareness of and participation in pancreas and islet cell transplantation. Methods: We analyzed Google Search trends for “Pancreas Transplantation” and “Islet Cell Transplantation” from 2004 to 14 November 2023, assessing variations in search interest over time and across geographical locations. The Augmented Dickey–Fuller (ADF) test was used to determine the stationarity of the trends (p < 0.05). Results: Search interest for “Pancreas Transplantation” varied from its 2004 baseline, with a general decline in peak interest over time. The lowest interest was in December 2010, with a slight increase by November 2023. Ecuador, Kuwait, and Saudi Arabia showed the highest search interest. “Islet Cell Transplantation” had its lowest interest in December 2016 and a more pronounced decline over time, with Poland, China, and South Korea having the highest search volumes. In the U.S., “Pancreas Transplantation” ranked 4th in interest, while “Islet Cell Transplantation” ranked 11th. The ADF test confirmed the stationarity of the search trends for both procedures. Conclusions: “Pancreas Transplantation” and “Islet Cell Transplantation” showed initial peaks in search interest followed by a general downtrend. The stationary search trends suggest a lack of significant fluctuations or cyclical variations. These findings highlight the need for enhanced educational initiatives to increase the understanding and awareness of these critical transplant procedures among the public and professionals.
Full article
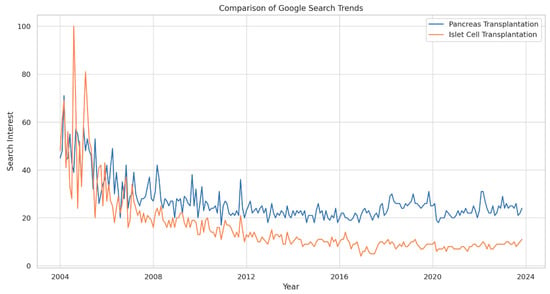
Figure 1
Open AccessArticle
Can Secondary Adhesive Capsulitis Complicate Calcific Tendinitis of the Rotator Cuff? An Ultrasound Imaging Analysis
by
Giovanni Tuè, Oriana Masuzzo, Francesco Tucci, Marco Cavallo, Anna Parmeggiani, Fabio Vita, Alberto Patti, Danilo Donati, Alessandro Marinelli, Marco Miceli and Paolo Spinnato
Clin. Pract. 2024, 14(2), 579-589; https://doi.org/10.3390/clinpract14020045 - 28 Mar 2024
Abstract
Background: Adhesive capsulitis (AC) of the glenohumeral joint is a recognized cause of pain associated with both active and passive restricted ranges of movement. AC can be subdivided into primary and secondary forms. Trauma, surgery, immobilization, and diabetes mellitus are the leading well-recognized
[...] Read more.
Background: Adhesive capsulitis (AC) of the glenohumeral joint is a recognized cause of pain associated with both active and passive restricted ranges of movement. AC can be subdivided into primary and secondary forms. Trauma, surgery, immobilization, and diabetes mellitus are the leading well-recognized causes of secondary AC. Calcific tendinitis/tendinitis (CT) of the rotator cuff is considered a possible trigger for AC, as reported in a few previous articles. However, there are no original investigations that assess the frequency and characteristics of this association. The aim of our research was to evaluate the presence of AC in a cohort of patients with a known CT condition of the rotator cuff by an ultrasound (US) examination. Materials and methods: We prospectively enrolled all the patients admitted at our single institution (October 2022–June 2023) for the preoperative US evaluation of a known CT condition. In these patients, we searched for parameters related to secondary AC. An axillary pouch (AP) thickness equal to or greater than 4 mm (or greater than 60% of the contralateral AP) was considered diagnostic of AC. Moreover, rotator interval (RI) thickness and the presence of effusion within the long-head biceps tendon (LHBT) sheath was also assessed in all patients. Results: A total of 78 patients (54F, 24M—mean age = 50.0 and range = 31–71 y.o.) were enrolled in the study. In 26 of those patients (26/78—33.3%), US signs of AC were detected. Notably, the mean AP thickness in patients with AC and CT was 3.96 ± 1.37 mm (Group 1) and 2.08 ± 0.40 mm in patients with CT only (Group 2). RI thickness was significantly greater in patients with superimposed AC: 2.54 ± 0.38 mm in Group 1 and 1.81 ± 0.41 mm in Group 2 (p < 0.00001). Moreover, effusion within the LHBT was significantly more frequently detected in patients with AC: 84.61% in Group 1 versus 15.79% in Group 2—p < 0.00001. Conclusion: US signs of AC are found in one-third of patients with CT of the rotator cuff, demonstrating that AC represents a frequent complication that should be routinely evaluated during US investigation to provide more personalized treatment strategies.
Full article
(This article belongs to the Special Issue Shoulder Disorders: Diagnosis and Treatment)
►▼
Show Figures
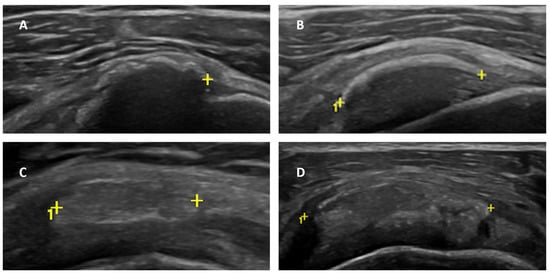
Figure 1
Open AccessArticle
Epidemiology of Hepatocellular Carcinoma in Taiwan
by
Yu-Wei Lai and Ching-Hu Chung
Clin. Pract. 2024, 14(2), 570-578; https://doi.org/10.3390/clinpract14020044 - 28 Mar 2024
Abstract
►▼
Show Figures
Background: Hepatocellular carcinoma (HCC) is a major contributor to the world’s cancer burden. Understanding the HCC incidence rate in Taiwan is thus an interesting avenue of research. Methods: From an NHI database, those patients who had been newly diagnosed with HCC and who
[...] Read more.
Background: Hepatocellular carcinoma (HCC) is a major contributor to the world’s cancer burden. Understanding the HCC incidence rate in Taiwan is thus an interesting avenue of research. Methods: From an NHI database, those patients who had been newly diagnosed with HCC and who had been listed on a registry in a catastrophic illness dataset during the years 2013–2021 were enrolled in this study. Antineoplastic agent usage and comorbidities were also studied. Results: The incidence rate of HCC decreased from 57.77 to 44.95 in 100,000 from 2013 to 2021. The average age of patients with HCC increased from 65.54 years old with a CCI score of 4.98 in 2013 to 67.92 years old with a CCI score of 5.49 in 2021. Among these HCC patients, the patients under antineoplastic agent treatment decreased from 53.47% to 31.41% from 2013 to 2021. The presence of comorbidities in HCC patients was about 55.77–83.01% with mild liver disease and 29.93–37.30% with diabetes (without complications) in the period 2013–2021. Conclusions: The incidence rate of HCC slightly decreased in Taiwan. Due to antineoplastic agent usage decreasing over time, these results may indicate that more early-stage HCC patients detected in recent years were mainly treated with surgeries.
Full article
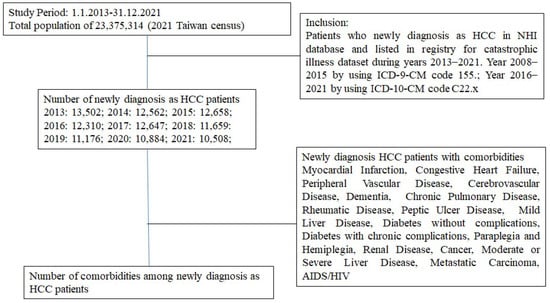
Figure 1
Open AccessArticle
Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance
by
Nicole Riberti, Emira D’Amico, Tania Vanessa Pierfelice, Michele Furlani, Alessandra Giuliani, Adriano Piattelli, Giovanna Iezzi and Luca Comuzzi
Clin. Pract. 2024, 14(2), 556-569; https://doi.org/10.3390/clinpract14020043 - 27 Mar 2024
Abstract
Background: In recent years, the use of conometric systems to connect dental implant abutments and prosthetic caps has been advocated because they seem to eliminate the side effects reported when using screw- and cement-connected prosthetic restorations. Objectives: The present case study
[...] Read more.
Background: In recent years, the use of conometric systems to connect dental implant abutments and prosthetic caps has been advocated because they seem to eliminate the side effects reported when using screw- and cement-connected prosthetic restorations. Objectives: The present case study is focused on conometric connection characterization and its performance in terms of the microarchitecture of peri-implant soft tissues by using a cross-linked approach based on optical microscopy and three-dimensional imaging. Methods: Two dental implants were characterized using micro-CT and another identical one was implanted into a patient; the latter was retrieved 45 days later due to changes in prosthetic needs. Afterward, the peri-implant soft tissues were investigated using synchrotron-based phase contrast imaging, histology, and polarized light microscopy. Results: Micro-CT analysis showed perfect adhesion between the abutment and prosthetic cap; histology and polarized light microscopy showed that connective tissue was richly present around the abutment retrieved from the patient. Moreover, the quantitative evaluation of connective tissues using synchrotron imaging, supported by artificial intelligence, revealed that this tissue was rich in mature collagen, with longitudinal and transverse collagen bundles intertwined. The number and connectivity of transverse bundles were consistently greater than those of the longitudinal bundles. Conclusion: It was found that the peri-implant soft tissue was already mature and well organized after only 45 days of implantation, supporting the hypothesis that conometric connections contribute to the significant stabilization of peri-implant soft tissues.
Full article
(This article belongs to the Special Issue New Trends, Materials, and Technologies and Consolidating Best Practices in Dentistry)
►▼
Show Figures
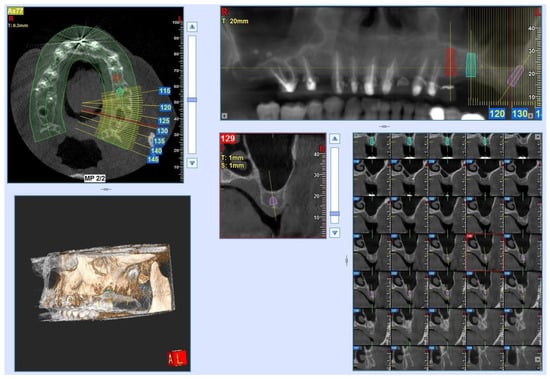
Figure 1
Open AccessArticle
The Influence of Wound Closure Techniques after Surgical Decompression in Patients with Carpal Tunnel Syndrome on Sleep Disturbance and Life Quality: A Prospective Comparison of Surgical Techniques
by
Veridijana Sunjic Roguljic, Luka Roguljic, Ivana Jukic and Vedran Kovacic
Clin. Pract. 2024, 14(2), 546-555; https://doi.org/10.3390/clinpract14020042 - 26 Mar 2024
Abstract
►▼
Show Figures
Background: The compression of the median nerve within the carpal tunnel is the cause of carpal tunnel syndrome (CTS). Surgical decompression is successful in improving sleep and quality of life, but the effect of tissue adhesives as a material for wound closure has
[...] Read more.
Background: The compression of the median nerve within the carpal tunnel is the cause of carpal tunnel syndrome (CTS). Surgical decompression is successful in improving sleep and quality of life, but the effect of tissue adhesives as a material for wound closure has not been investigated. The objective of the study was to evaluate sleep disorders and health-related life quality by comparing two methods for wound closure after carpal surgery in participants who were randomized to receive tissue adhesives or transcutaneous sutures. Methods: The subjects, aged 61.56 ± 12.03 years, were randomized to receive either tissue adhesives (n = 50) or suture-based wound closure (n = 50) using the Glubran Tiss 2® skin adhesive after subcutaneous running sutures. The outcomes were assessed during the 12-month postoperative follow-up. The Pittsburgh Sleep Quality Index (PQSI) and Insomnia Severity Scale (ISI) were used for the sleep disturbance assessment, and for the health-related quality of life assessment, the total SF-36 (36-Item Short Form Survey) was used. Results: The PQSI, ISI, and SF-36 were not statistically different between groups during the follow-up, except in the ISI score two weeks after surgery (9.40 ± 1.18 in the tissue adhesive group vs. 9.96 ± 1.09 in the suture-based group, p = 0.008). The PQSI, ISI, and SF-36 scores for all the subjects and groups were persistently improved at all the follow-up intervals after surgery. The total SF-36 score increased 12 months after surgery (49.84 ± 5.85 vs. 82.46 ± 5.68, p < 0.001). Conclusions: Cyanoacrylate-based adhesion material can be used for wound closure after open CTS decompression as a standard transcutaneous suture, and both techniques equally lead to improved sleep and life quality. The possible advantages of tissue adhesives include a faster reduction in the ISI.
Full article
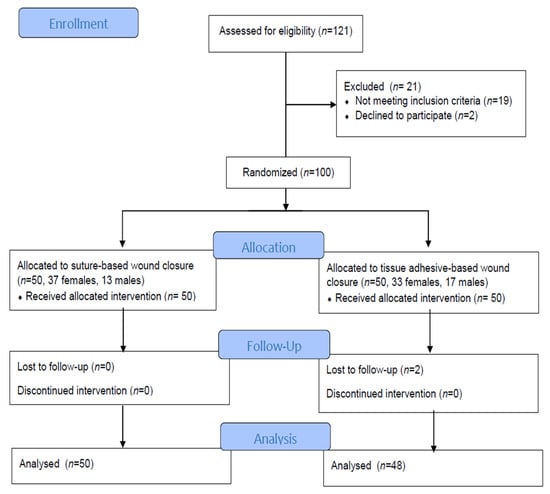
Figure 1
Open AccessArticle
Comparisons of the Rates of Large-for-Gestational-Age Newborns between Women with Diet-Controlled Gestational Diabetes Mellitus and Those with Non-Gestational Diabetes Mellitus
by
Sirida Pittyanont, Narongwat Suriya, Sirinart Sirilert and Theera Tongsong
Clin. Pract. 2024, 14(2), 536-545; https://doi.org/10.3390/clinpract14020041 - 25 Mar 2024
Abstract
(1) Objectives: The primary objective is to compare the rate of large-for-gestational-age (LGA) between women with diet-controlled gestational diabetes mellitus (GDM) and those with non-GDM, and to assess whether or not diet-controlled GDM is an independent factor of LGA fetuses. The secondary
[...] Read more.
(1) Objectives: The primary objective is to compare the rate of large-for-gestational-age (LGA) between women with diet-controlled gestational diabetes mellitus (GDM) and those with non-GDM, and to assess whether or not diet-controlled GDM is an independent factor of LGA fetuses. The secondary objectives are to compare the rates of other common adverse pregnancy outcomes, such as preeclampsia, cesarean section rate, preterm birth, and low Apgar score, between pregnancies with diet-controlled GDM and non-GDM pregnancies. (2) Methods: A retrospective cohort study was conducted on singleton pregnancies, diagnosed with GDM and non-GDM between 24 and 28 weeks of gestation, based on a two-step screening test. The prospective database of the obstetric department was accessed to retrieve the records meeting the inclusion criteria, and full medical records were comprehensively reviewed. The patients were categorized into two groups, GDM (study group) and non-GDM (control group). The main outcome was the rate of LGA newborns, and the secondary outcomes included pregnancy-induced hypertension, preterm birth, cesarean rate, low Apgar scores, etc. (3) Results: Of 1364 recruited women, 1342 met the inclusion criteria, including 1177 cases in the non-GDM group and 165 (12.3%) in the GDM group. Maternal age and pre-pregnancy BMI were significantly higher in the GDM group. The rates of LGA newborns, PIH, and cesarean section were significantly higher in the GDM group (15.1% vs. 7.1%, p-value < 0.001; 7.8% vs. 2.6%, p-value = 0.004; and 54.5% vs. 41.5%, p-value = 0.002; respectively). On logistic regression analysis, GDM was not significantly associated with LGA (odds ratio 1.64, 95% CI: 0.97–2.77), while BMI and gender were still significantly associated with LGA. Likewise, GDM was not significantly associated with the rate of PIH (odds ratio: 1.7, 95% CI: 0.825–3.504), while BMI and maternal age were significantly associated with PIH, after controlling confounding factors. (4) Conclusions: The rates of LGA newborns, PIH, and cesarean section are significantly higher in women with diet-controlled GDM than those with non-GDM. Nevertheless, the rates of LGA newborns and PIH are not directly caused by GDM but mainly caused high pre-pregnancy BMI and advanced maternal age, which are more commonly encountered among women with GDM.
Full article
(This article belongs to the Special Issue Clinical Nutrition in Metabolic Disorders)
Open AccessReview
Magnesium Is a Vital Ion in the Body—It Is Time to Consider Its Supplementation on a Routine Basis
by
Ákos Géza Pethő, Tibor Fülöp, Petronella Orosz and Mihály Tapolyai
Clin. Pract. 2024, 14(2), 521-535; https://doi.org/10.3390/clinpract14020040 - 22 Mar 2024
Abstract
►▼
Show Figures
The importance of maintaining proper magnesium intake and total body magnesium content in preserving human health remains underappreciated among medical professionals and laymen. This review aimed to show the importance of hypomagnesemia as a modifiable risk factor for developing disease processes. We searched
[...] Read more.
The importance of maintaining proper magnesium intake and total body magnesium content in preserving human health remains underappreciated among medical professionals and laymen. This review aimed to show the importance of hypomagnesemia as a modifiable risk factor for developing disease processes. We searched the PubMed database and Google Scholar using the keywords ‘magnesium’, ‘diabetes’, ‘cardiovascular disease’, ‘respiratory disease’, ‘immune system’, ‘inflammation’, ‘autoimmune disease’, ‘neurology’, ‘psychiatry’, ‘cognitive function’, ‘cancer’, and ‘vascular calcification’. In multiple contexts of the search terms, all reviews, animal experiments, and human observational data indicated that magnesium deficiency can lead to or contribute to developing many disease states. The conclusions of several in-depth reviews support our working hypothesis that magnesium and its supplementation are often undervalued and underutilized. Although much research has confirmed the importance of proper magnesium supply and tissue levels, simple and inexpensive magnesium supplementation has not yet been sufficiently recognized or promoted.
Full article
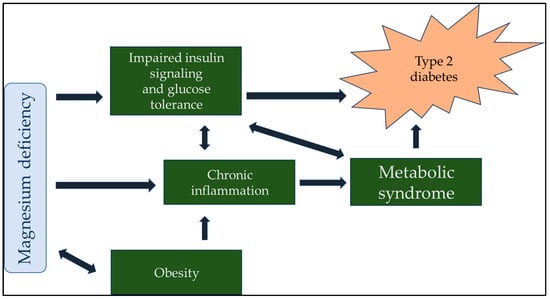
Figure 1
Open AccessArticle
Tissue-Based Genomic Testing in Prostate Cancer: 10-Year Analysis of National Trends on the Use of Prolaris, Decipher, ProMark, and Oncotype DX
by
Eugenio Bologna, Francesco Ditonno, Leslie Claire Licari, Antonio Franco, Celeste Manfredi, Spencer Mossack, Savio Domenico Pandolfo, Cosimo De Nunzio, Giuseppe Simone, Costantino Leonardo and Giorgio Franco
Clin. Pract. 2024, 14(2), 508-520; https://doi.org/10.3390/clinpract14020039 - 19 Mar 2024
Abstract
Background: Prostate cancer (PCa) management is moving towards patient-tailored strategies. Advances in molecular and genetic profiling of tumor tissues, integrated with clinical risk assessments, provide deeper insights into disease aggressiveness. This study aims to offer a comprehensive overview of the pivotal genomic tests
[...] Read more.
Background: Prostate cancer (PCa) management is moving towards patient-tailored strategies. Advances in molecular and genetic profiling of tumor tissues, integrated with clinical risk assessments, provide deeper insights into disease aggressiveness. This study aims to offer a comprehensive overview of the pivotal genomic tests supporting PCa treatment decisions, analyzing—through real-world data—trends in their use and the growth of supporting literature evidence. Methods: A retrospective analysis was conducted using the extensive PearlDiver™ Mariner database, which contains de-identified patient records, in compliance with the Health Insurance Portability and Accountability Act (HIPAA). The International Classification of Diseases (ICD) and Current Procedural Terminology (CPT) codes were employed to identify patients diagnosed with PCa during the study period—2011 to 2021. We determined the utilization of primary tissue-based genetic tests (Oncocyte DX®, Prolaris®, Decipher®, and ProMark®) across all patients diagnosed with PCa. Subsequently, within the overall PCa cohort, patients who underwent radical prostatectomy (RP) and received genetic testing postoperatively were identified. The yearly distribution of these tests and the corresponding trends were illustrated with graphs. Results: During the study period, 1,561,203 patients with a PCa diagnosis were recorded. Of these, 20,748 underwent tissue-based genetic testing following diagnosis, representing 1.3% of the total cohort. An increasing trend was observed in the use of all genetic tests. Linear regression analysis showed a statistically significant increase over time in the use of individual tests (all p-values < 0.05). Among the patients who underwent RP, 3076 received genetic analysis following surgery, representing 1.27% of this group. Conclusions: Our analysis indicates a growing trend in the utilization of tissue-based genomic testing for PCa. Nevertheless, they are utilized in less than 2% of PCa patients, whether at initial diagnosis or after surgical treatment. Although it is anticipated that their use may increase as more scientific evidence becomes available, their role requires further elucidation.
Full article
(This article belongs to the Special Issue Urologic Oncology: Recent Advances and Future Perspectives)
►▼
Show Figures
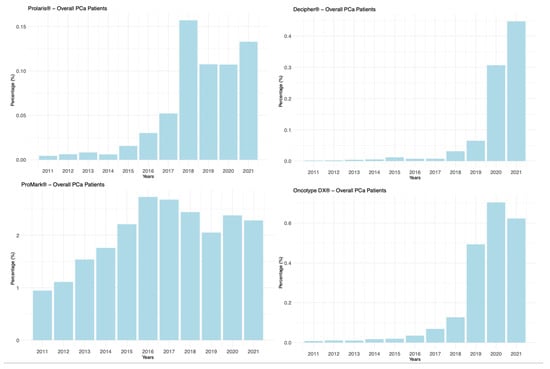
Figure 1
Open AccessArticle
The Role of Illness Perception and Self-Efficacy in Determining Quality of Life among Cancer Patients
by
Aisha Alhofaian, Marym M. Alaamri, Mariam Ahmed Abdalmajeed, Lama Saeed Wadaah, Leena Abdulaziz Aljuhani, Meral Ammar Amin, Afnan Tunsi and Ruba Alharazi
Clin. Pract. 2024, 14(2), 498-507; https://doi.org/10.3390/clinpract14020038 - 19 Mar 2024
Abstract
Background: The quality of life for people with chronic illnesses like cancer has been shown to be significantly impacted by self-efficacy and perceptions of their illness. Objectives: This study investigates the relationship between cancer patients’ perceptions of their illness, their self-efficacy beliefs, and
[...] Read more.
Background: The quality of life for people with chronic illnesses like cancer has been shown to be significantly impacted by self-efficacy and perceptions of their illness. Objectives: This study investigates the relationship between cancer patients’ perceptions of their illness, their self-efficacy beliefs, and their quality of life. Method: Conducted from December 2022 to February 2023, this research involved 120 adults undergoing cancer treatment. We utilized the Illness Perception Questionnaire (IPQ), the Arabic version of the Cancer Behavioral Inventory Brief (CBI-B), and the Arabic EORTC QLQ-C30, alongside clinical data collection. Statistical analyses included Pearson correlation and descriptive statistics. Results: Breast cancer emerged as the most common type among participants. A positive correlation was found between self-efficacy and quality of life, as measured by the EORTC QLQ-C30, particularly in relation to symptom management. Interestingly, all dimensions of illness perception correlated with quality of life, except for control and concerns. Conclusions: The findings underscore the vital role of nurses and healthcare providers in aiding cancer patients to develop and utilize self-management strategies effectively. The study reveals that a patient’s capacity to manage their illness is significantly influenced by their confidence, understanding of their condition, and overall quality of life. Addressing these aspects can greatly enhance healthcare professionals’ contribution to improving the resilience and well-being of individuals battling cancer.
Full article
Open AccessArticle
Anxiety and Depression and Associated Risk Factors among Outpatients with Systemic Lupus Erythematosus: Eastern Province, Saudi Arabia
by
Manal Ahmed Hasan, Wasayf Salman Almogaliq, Fatimah Habib Alhanabi, Hebah Abbas Aldrazi, Moath Thamer Alkhouzaie, Raed Albukhari, Safi Alqatari, Abdullah A. Al-Abdulwahab, Hajer Musaab AlZuhair and Mohammed T. Al-Hariri
Clin. Pract. 2024, 14(2), 486-497; https://doi.org/10.3390/clinpract14020037 - 19 Mar 2024
Abstract
Background: Although mood disorders are prevalent among systemic lupus erythematosus (SLE) patients, they are usually underrecognized. This study aimed to estimate the prevalence of anxiety and depression among Saudi SLE patients. Methods: This cross-sectional study was conducted among SLE patients from July 2022
[...] Read more.
Background: Although mood disorders are prevalent among systemic lupus erythematosus (SLE) patients, they are usually underrecognized. This study aimed to estimate the prevalence of anxiety and depression among Saudi SLE patients. Methods: This cross-sectional study was conducted among SLE patients from July 2022 to June 2023 in the Eastern Province of Saudi Arabia. A self-reported questionnaire was used to collect the data through validated tools including the Hamilton Anxiety Rating Scale-A and the Beck Depression Inventory score. Results: There were 133 females (91.7%) and 12 males (8.3%) included in this study. Based on the HAM-A score, 45.5% of participants had an anxiety disorder, and according to the BDI score, 46.2% had a depression disorder. Anxiety and depression were significantly associated with a longer duration of SLE, unemployment status, smoking, and the presence of comorbidities. Moreover, the present study found a significant association between depression and male gender. Conclusion: This study found that Saudi SLE patients have a high prevalence of both anxiety and depression. Therefore, SLE patients should be screened for neuropsychiatric disorders during routine follow-ups and managed as early as possible.
Full article
(This article belongs to the Special Issue Pediatric and Adolescent Psychiatry and Psychology: Current Treatments and Future Options)
►▼
Show Figures
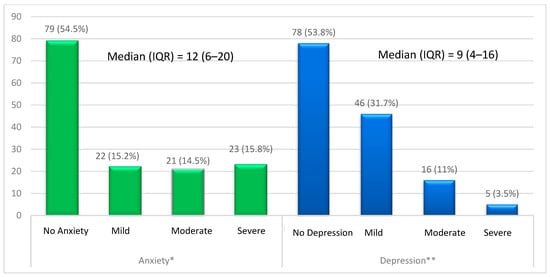
Figure 1
Open AccessArticle
Breast Cancer Risk Factors among Women with Solid Breast Lesions
by
Ivana Eremici, Andreea Borlea, Catalin Dumitru and Dana Stoian
Clin. Pract. 2024, 14(2), 473-485; https://doi.org/10.3390/clinpract14020036 - 18 Mar 2024
Abstract
►▼
Show Figures
Background: Breast cancer is the most frequent malignancy in women worldwide and one of the most curable cancers if diagnosed at an early stage. Female patients presenting solid breast lesions are greatly predisposed to breast cancer development, and as such, effective screening of
[...] Read more.
Background: Breast cancer is the most frequent malignancy in women worldwide and one of the most curable cancers if diagnosed at an early stage. Female patients presenting solid breast lesions are greatly predisposed to breast cancer development, and as such, effective screening of high-risk patients is valuable in early-stage breast cancer detection. Objectives: The aim of our study was to identify the most relevant demographic, reproductive and lifestyle risk factors for breast cancer among women with solid breast lesions living in western Romania, namely the urban region consisting of Timisoara and the rural surrounding regions. Methods: From January 2017 to December 2021, 1161 patients with solid breast lesions, as detected by sonoelastography, were divided into two groups: patients with benign lesions (1019, 87.77%) and patients with malignant nodules (142, 12.23%). The malignancy group was confirmed by a histopathological result. Variables including age, BMI, menarche, menopause, years of exposure to estrogen, number of births, breastfeeding period, use of oral combined contraceptives, smoker status, family medical history and living area (rural-urban) were recorded. Results: It was evidenced by our study that the main risk factors for malignancy were elevated age (OR = 1.07, 95% CI 1.05–1.08), BMI (OR = 1.06, 95% CI 1.02–1.10), living area (rural) (OR = 1.86, 95% CI 1.13–2.85) and family medical history (negative) (OR 3.13, 95% CI 1.43–8.29). The other proposed risk factors were not found to be statistically significant. Conclusions: Age and BMI were observed to be the most significant factors for breast cancer risk increase, followed by living in a rural area. A family history of breast cancer was shown to be inversely correlated with cancer risk increase.
Full article
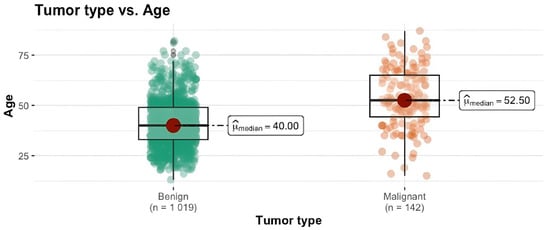
Figure 1
Open AccessSystematic Review
Celecoxib Decreases the Need for Rescue Analgesics after Total Knee Arthroplasty: A Meta-Analysis
by
Eduardo Gómez-Sánchez, Adriana Hernández-Gómez, Juan Manuel Guzmán-Flores, Angel Josabad Alonso-Castro, Nicolás Addiel Serafín-Higuera, Luz Ma.-Adriana Balderas-Peña, Lorenzo Franco-de la Torre and Mario Alberto Isiordia-Espinoza
Clin. Pract. 2024, 14(2), 461-472; https://doi.org/10.3390/clinpract14020035 - 18 Mar 2024
Abstract
This systematic review and meta-analysis aimed to evaluate the analgesic efficacy and adverse effects of celecoxib after total knee arthroplasty. Keywords in the PubMed and Scopus databases were used to find article abstracts. Each included clinical trial was assessed using the Cochrane Collaboration
[...] Read more.
This systematic review and meta-analysis aimed to evaluate the analgesic efficacy and adverse effects of celecoxib after total knee arthroplasty. Keywords in the PubMed and Scopus databases were used to find article abstracts. Each included clinical trial was assessed using the Cochrane Collaboration risk of bias tool, and we extracted data on postoperative pain assessment using the Visual Analogue Scale (VAS) at rest, ambulation, and active range of motion, rescue analgesic intake, and adverse effects. Inverse variance tests with mean differences were used to analyze the numerical variables. The Mantel–Haenszel statistical method and the odds ratio were used to evaluate the dichotomous data. According to this qualitative assessment (n = 482), two studies presented conclusions in favor of celecoxib (n = 187), one showed similar results between celecoxib and the placebo (n = 44), and three clinical trials did not draw conclusions as to the effectiveness of celecoxib versus the placebo (n = 251). Moreover, the evaluation of the rescue analgesic intake showed that the patients receiving celecoxib had a lower intake compared to patients receiving a placebo (n = 278, I2 = 82%, p = 0.006, mean difference = −6.89, 95% IC = −11.76 to −2.02). In conclusion, the pooled analysis shows that administration of celecoxib alone results in a decrease in rescue analgesic consumption compared to a placebo after total knee surgery.
Full article
(This article belongs to the Special Issue 2023 Feature Papers in Clinics and Practice)
►▼
Show Figures
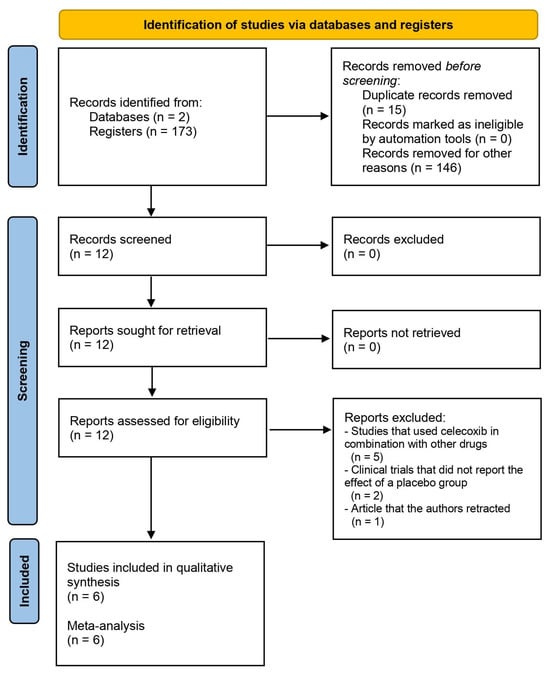
Figure 1
Open AccessArticle
Health Patterns across Adulthood: An Age-Based Investigation of the Nutritional Status, Homocysteine, and CoQ10 of Bank Staff
by
Markus Schauer, Susanne Mair, Mohamad Motevalli, Derrick Tanous, Martin Burtscher and Katharina Wirnitzer
Clin. Pract. 2024, 14(2), 443-460; https://doi.org/10.3390/clinpract14020034 - 14 Mar 2024
Abstract
Background: This study aimed to evaluate age-specific variations in the blood levels of micronutrients, homocysteine, and CoQ10, along with physical activity (PA) patterns, among 123 Austrian adult bankers in operational and frontline roles (mean age: 43 years; 50% female). Methods: Blood analysis was
[...] Read more.
Background: This study aimed to evaluate age-specific variations in the blood levels of micronutrients, homocysteine, and CoQ10, along with physical activity (PA) patterns, among 123 Austrian adult bankers in operational and frontline roles (mean age: 43 years; 50% female). Methods: Blood analysis was conducted to assess micronutrients and the serum concentrations of homocysteine and CoQ10. The micronutrient values in whole blood were compared to sex-specific reference ranges and categorized as below, within, or above them. The Global Physical Activity Questionnaire was utilized to assess PA patterns. Participants were classified as young adults (18–34 years), middle-aged adults (35–49 years), and older adults (50–64 years). Results: Significant age-based differences were found in participants’ mean homocysteine levels (p = 0.039) and homocysteine categories (p = 0.034), indicating an increasing prevalence of hyperhomocysteinemia with age. No significant difference between age categories was observed for sex, BMI, diet types, PA levels, sedentary behavior, and CoQ10 (p > 0.05). There was no significant age-based difference in the blood concentrations of most minerals and vitamins (p > 0.05), except for magnesium among females (p = 0.008) and copper among males (p = 0.042). Conclusion: The findings offer initial evidence of the age-related differences in the health status of adult bankers, providing insights for customized approaches to occupational health that support the importance of metabolic health and overall well-being across adulthood.
Full article
(This article belongs to the Special Issue Clinical Nutrition in Metabolic Disorders)
►▼
Show Figures
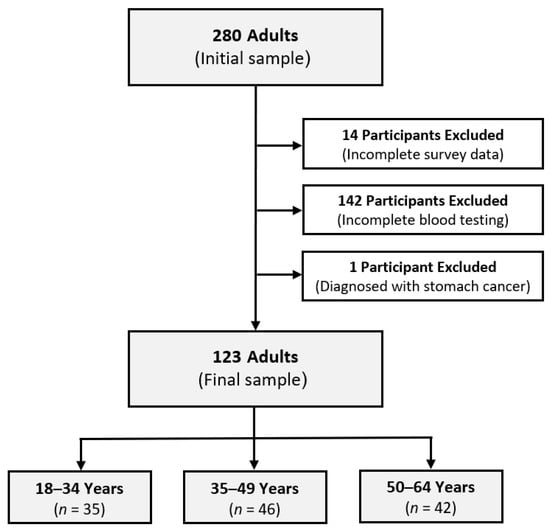
Figure 1
Open AccessCase Report
Diagnostic and Therapeutic Challenges of Oligosymptomatic Vesicovaginal Fistula in the Complex Case of Endometriosis
by
Agnieszka A. Strojny, Arkadiusz Baran, Katarzyna Wiejak, Anna Scholz and Radosław B. Maksym
Clin. Pract. 2024, 14(2), 436-442; https://doi.org/10.3390/clinpract14020033 - 12 Mar 2024
Abstract
Endometriosis is a complex condition causing surgical challenges, sometimes leading to urogynecological complications, the diagnosis and treatment of which are not always obvious. We present a case of a 46-year-old woman with a history of severe endometriosis and adenomyosis who developed an oligosymptomatic
[...] Read more.
Endometriosis is a complex condition causing surgical challenges, sometimes leading to urogynecological complications, the diagnosis and treatment of which are not always obvious. We present a case of a 46-year-old woman with a history of severe endometriosis and adenomyosis who developed an oligosymptomatic vesicovaginal fistula (VVF) as a complication of surgery. The patient’s medical history included multiple surgeries for endometriosis, a cesarean section, and a laparoscopic hysterectomy. After the excision of the full-thickness infiltration of the urinary bladder, she experienced postoperative bowel obstruction treated by laparotomy. Subsequent urinary complications of bladder healing were eventually recognized as oligosymptomatic VVF. Symptoms of VVFs may vary, making a diagnosis challenging, especially when the lesion is narrow. Imaging techniques such as cystoscopy and cystography are helpful for diagnosis. The treatment options for VVFs range from surgical repair to conservative methods, like bladder catheterization, hormonal therapy, and platelet-rich plasma (PRP) injections, depending on the lesions’ size and location. In this case, the patient’s VVF was treated with PRP injections, a low-invasive method in urogynecology. PRP, known for its pleiotropic role, is increasingly used in medicine, including gynecology. The patient’s fistula closed after 6 weeks from the PRP session, highlighting the potential of this conservative treatment modality.
Full article
(This article belongs to the Topic Pathology and Current State of Treatment of Chronic Pain)
►▼
Show Figures
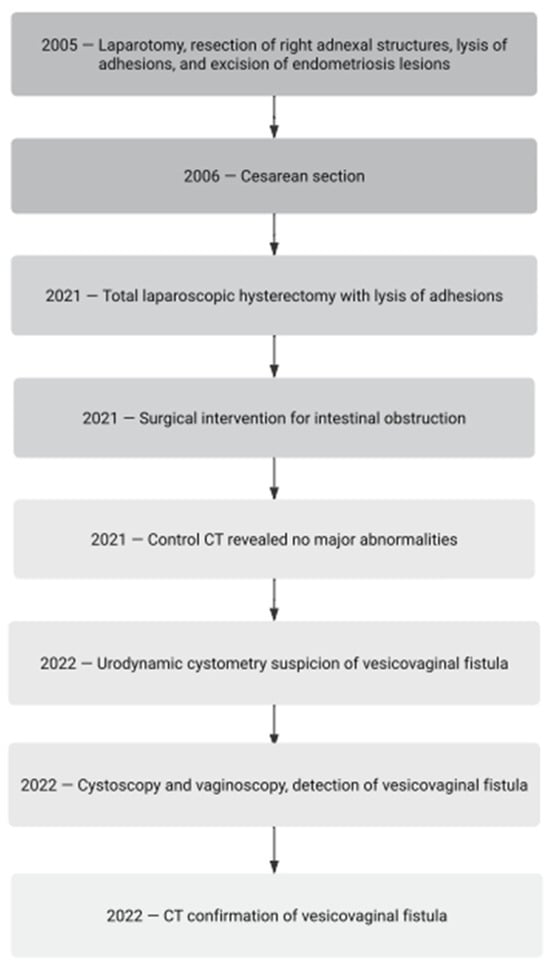
Figure 1
Open AccessArticle
The Relationship between Vitamin D Levels and Blood Glucose and Cholesterol Levels
by
Eman Elsheikh, Abdulhakim Ibrahim Alabdullah, Sarah Saleh Al-Harbi, Amal Omar Alagha, Dhiyaa Hassan AlAhmed and Mazen Moraya Ali Alalmaee
Clin. Pract. 2024, 14(2), 426-435; https://doi.org/10.3390/clinpract14020032 - 29 Feb 2024
Abstract
Background: Vitamin D deficiency has reached epidemic proportions globally. Observational data link low vitamin D status to diabetes, dyslipidemia, and metabolic syndrome, but interventional trials on the effects of supplementation are limited. Objective: We investigated associations between serum 25-hydroxyvitamin D (25(OH)D) levels and
[...] Read more.
Background: Vitamin D deficiency has reached epidemic proportions globally. Observational data link low vitamin D status to diabetes, dyslipidemia, and metabolic syndrome, but interventional trials on the effects of supplementation are limited. Objective: We investigated associations between serum 25-hydroxyvitamin D (25(OH)D) levels and metabolic markers in Saudi adults. Methods: This retrospective cross-sectional study analyzed the clinical records of 476 patients from Saudi Arabia, aged 15–78 years. According to 25(OH)D levels, participants were stratified as vitamin D-sufficient (≥30 ng/mL), -insufficient (21–29 ng/mL), or -deficient (≤20 ng/mL). The outcomes were diabetic status (fasting glucose, HbA1c) and lipid panel results. Results: Higher diabetes prevalence was significantly associated with lower 25(OH)D levels (10.1% in the sufficient group, 11.6% in the insufficient group, and 18.3% in the deficient group). Similarly, worse lipid profiles were associated with more severe hypovitaminosis D, including a total cholesterol level of ≥240 mg/dL (5.3% in participants with normal vitamin D levels vs. 18.9% in those with deficient levels) and LDL ≥ 160 mg/dL (6.9% in participants with normal vitamin D levels vs. 13.2% in those with deficient levels). Vitamin D deficiency disproportionately affected women and adults > 45 years old. Conclusions: Vitamin D deficiency is endemic in Saudi Arabia and strongly linked to worsened metabolic markers. Optimizing vitamin D status through screening and correcting the deficiency may provide a cost-effective approach to confronting the regional diabetes epidemic and reducing cardiovascular disease risk.
Full article
(This article belongs to the Special Issue Clinical Nutrition in Metabolic Disorders)
Open AccessArticle
Candida Variety in the Oral Cavity of Mexican Subjects with Type 2 Diabetes Mellitus and TLR2 Gene Expression
by
Nadia Mabel Pérez-Vielma, Modesto Gómez-López, María de los Ángeles Martínez-Godínez, Ana Laura Luna-Torres, Aarón Domínguez López and Ángel Miliar-García
Clin. Pract. 2024, 14(2), 417-425; https://doi.org/10.3390/clinpract14020031 - 27 Feb 2024
Abstract
►▼
Show Figures
Background: The aim was to diagnose Candida in the oral cavity of subjects with type 2 diabetes mellitus (T2DM) using a genotyping technique and compare the results with those from conventional diagnosis by Papanicolaou (Pap) staining. Methods: Palatal mucosa smears were performed on
[...] Read more.
Background: The aim was to diagnose Candida in the oral cavity of subjects with type 2 diabetes mellitus (T2DM) using a genotyping technique and compare the results with those from conventional diagnosis by Papanicolaou (Pap) staining. Methods: Palatal mucosa smears were performed on 18 dental care patients diagnosed with T2DM and grade I, II, and III prosthetic stomatitis who met the inclusion criteria; 18 healthy control subjects were also included in the study. Hemoglobin A1c (HbA1c) levels were determined from total blood. Using exfoliative cytology, the Pap staining technique was used to diagnose candidiasis. Exfoliative cytology was also used for molecular diagnosis; DNA was obtained for Candida genotyping, and RNA was used for gene expression studies. Results: Clinical patterns indicated that all subjects were positive for Candida; however, Pap analysis revealed only three positive subjects, whereas end-point polymerase chain reaction (PCR) analysis revealed 15 subjects with some type of Candida. The most common Candida species found were Candida guilliermondii (38.8%), Candida krusei (33.3%), Candida tropicalis, and Candida lusitaniae (22.2%). Interestingly, the coexpression of different species of Candida was found in various patients. In all patients, HbA1c levels were increased. Gene expression analysis showed a significant decrease (p ≤ 0.05) in TLR2 expression in positive subjects, whereas TLR4 expression did not differ significantly among patients. Conclusions: The end-point PCR technique showed better sensitivity for the diagnosis of Candida when compared with the diagnosis by Pap staining. T2DM subjects showed an increased presence of C. guilliermondii that was correlated with decreased TLR2 expression.
Full article
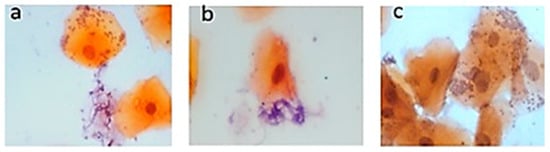
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Clinics and Practice, JCM, JPM, Healthcare, Diagnostics
Pathology and Current State of Treatment of Chronic Pain
Topic Editors: Tatsunori Ikemoto, Young-Chang AraiDeadline: 31 July 2024
Topic in
Biomedicines, Cancers, Clinics and Practice, Dermato, Life
Epidemiology and Risk Factors of Skin Cancer
Topic Editors: José Juan Pereyra-Rodríguez, Ricardo Ruiz-Villaverde, Jose-Carlos Armario-HitaDeadline: 30 September 2024
Topic in
Biomedicines, Clinics and Practice, Diagnostics, JCM, Rheumato
Rheumatic Disorder: From Basic Science to Clinical Practice
Topic Editors: Giulia Cassone, Caterina Vacchi, Andreina ManfrediDeadline: 20 December 2024
Topic in
Healthcare, Pharmacy, Clinics and Practice, Nursing Reports, EJIHPE
Advancing the Knowledge and Application of Health Behavior Theories
Topic Editors: Yifei Liu, Dhananjay NayakankuppamDeadline: 31 December 2024

Conferences
Special Issues
Special Issue in
Clinics and Practice
Clinical Outcome Research in the Head and Neck
Guest Editors: Ioannis Tilaveridis, Athanassios A. KyrgidisDeadline: 20 April 2024
Special Issue in
Clinics and Practice
Clinical Nutrition in Metabolic Disorders
Guest Editors: Charalampia Amerikanou, Aristea Gioxari, Efstathia PapadaDeadline: 15 May 2024
Special Issue in
Clinics and Practice
Clinical Practice of Artificial Intelligence in Diagnostic and Treatment Assistance
Guest Editors: Wisit Cheungpasitporn, Charat ThongprayoonDeadline: 31 May 2024
Special Issue in
Clinics and Practice
Management of Thyroid Diseases: Current Strategies and Future Directions
Guest Editor: Fausto FamàDeadline: 30 June 2024